An Innovation Pathway of Suppository Molds: Problems, Solutions and Comparisons
An Innovation Pathway of Suppository Molds: Problems,
Solutions and Comparisons
by Süsen Gülce Erismis, BPharm MSc.
Definitions and History
The term ‘insert’ refers a semi-solid pharmaceutical dosage form
carrying active pharmaceutical ingredients (APIs) dispersed in a base that
melts at body temperature or dissolves, applied by placing into naturally
occurring body cavities aside from the mouth or rectum, such as the vagina (1).
If the insert is intended for insertion into the vagina, it is called a vaginal
insert, vaginal suppository, or pessary (2). On the other hand, if the dosage
form is prepared for administration into the rectum, it is called a suppository
(3).
Due to their numerous advantages, both vaginal inserts and suppositories
have been widely prepared in pharmacies since the 18th century to
achieve both systemic and local effects. The first suppositories were molded
into a paper cone. However paper was not the best material to mold a
suppository especially considering wettability. Therefore, metal suppository
molds made from pewter and tin were introduced in 1860. Since the first mold
was a one-piece model, it was very difficult to remove the suppositories from
the cavities. For this reason, two-piece mold models have been designed to
facilitate the removal of the suppository while maintaining its shape (4). With
the developments in material science over the years, various two-piece suppository
molds have been produced using different metal combinations and are still in
use today.
Problems, Solutions and Comparisons
The metal-based suppository molds are known for their durability due to
their composition of brass, steel or anodized aluminum. For easy removal of the
suppositories from the mold and to maintain the shape, the mold should be
lubricated with a thin layer of a lubricant such as liquid paraffin before pouring
the preparation into the mold. However, excessive use of lubricant should be
avoided, otherwise excess lubricant may accumulate at the bottom of the
suppository mold and cause homogeneity, dosage and application problems. In
addition, the metal suppository molds are too expensive, and they can cause fractures
and fissures throughout the suppositories if the mold is too cold while
opening. To overcome these problems, different suppository molds have been
designed with more affordable materials.
Suppository molds, which are most similar to metal molds in terms of
material structure, are made of hard rubber. Similar to metal molds, they can
be designed in two parts to be opened by separating from the center or in one-piece
unlike metal molds. The one-piece rubber suppository molds contain a cap at the
bottom of the mold and the suppositories can be taken out by pushing through
the top after removing the cap. Although they provide a more affordable
solution than metal molds, there is a risk of fracture of the suppositories
when opening the lid.
Flexible rubber is another material that is used make one-piece
suppository molds. After the suppository mixture has solidified, it is removed
from the mold by pushing it through the back of each cavity, thus eliminating
the risk of fracture. These types of molds are also suitable for the
suppositories that need to be stored in the fridge (5). All of those re-usable
suppository molds require a convenient packaging to protect the compounded
product.
Primary Packaging Regulations
Packaging is a crucial step of the compounding process to maintain the
stability and quality of the preparation throughout its shelf life. According
to the World Health Organization (WHO), the package must protect the
pharmaceuticals against microbial contamination, physical damage, and external
influences such as oxygen, moisture and light. They also point out that there
should be no interaction, affecting the protective function, between the
packaging material and pharmaceutical (6). Also, Good Manufacturing Practice (GMP)
of ISO 15378:2017 states that production and control of primary packaging
materials are important for the safety of a patient receiving the medicinal
product because of the direct contact between the packaging materials and the
product (7).
Traditionally, the suppositories are wrapped individually in foil
(usually aluminum) as a primary packaging material and then, they are put into a
jar as secondary package. However, foil is not an ideal form of packaging for
protecting suppositories in accordance with WHO recommendations, as it tears
easily. Therefore, there is a need for more convenient solutions for the
pharmaceutical packaging of suppositories.
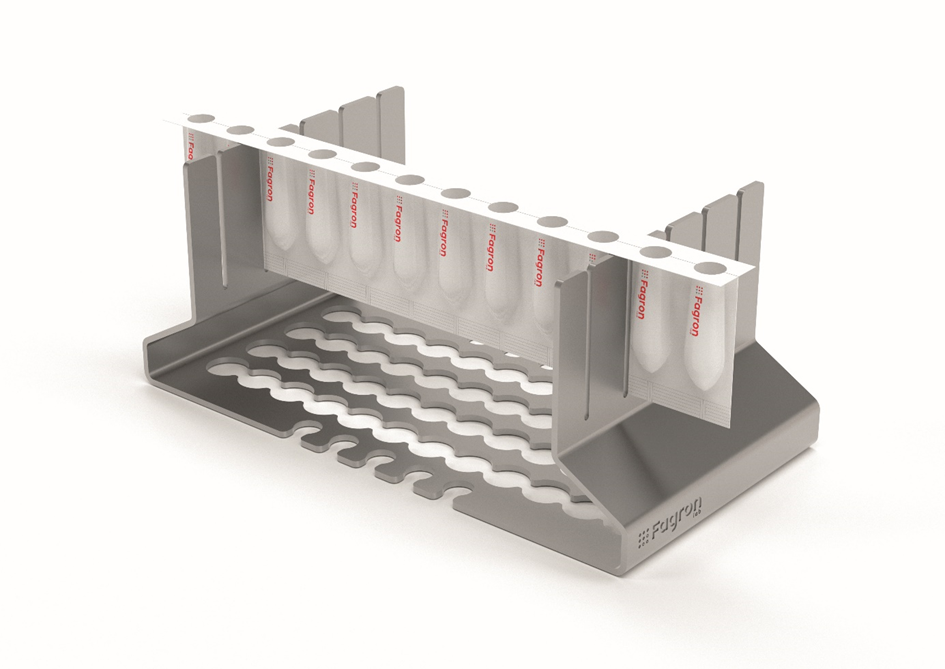
New Pursuits: FagronLab™ Suppository
Strips
FagronLab™ Suppository Strips are disposable molds that are also used as
the final packaging. The suppositories are removed from the mold by the patient
peeling the two halves of the mold apart from the bottom. There is no need for
the suppositories to be opened and re-packaged after preparation in the
pharmacy, thus they are a more hygienic and practical solution, fulfilling the
WHO recommendations, to deliver the inserts to the patients.
FagronLab™ Suppository Strips are composed from polyvinyl chloride (PVC)
material coated with polyvinylidene chloride (PVDC), reducing the permeability
of the film to oxygen, moisture and light, thus offering a stable packaging
option for the duration of the compounded beyond use date. They also have high
chemical resistance to alkalis, acids and organic solvents and are a safe
primary package as specified in the GMP guideline. This feature also eliminates
the need of using lubricants during preparation, preventing complication in
case of excessive lubricant usage.
The strips are resistant to tearing during transportation, eliminating
the risk of contamination caused by packaging material or cross-contamination
with the environment. Moreover, they are cost-efficient and valuable tools for
the compounding pharmacy, eliminating the need to purchase additional
packaging. For more information, reach out to the Fagron Academy team at facts.support@Fagronacademy.us.
Suppository strip tape allows for easy sealing of suppositories and negates
the need for heat sealing.
References
1.
NCI
Thesaurus [Internet]. ncithesaurus.nci.nih.gov. [cited 2023 Feb 22]. Available
from: https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&version=23.01e&ns=ncit&code=C60933&key=644923653&b=1&n=null
2.
NCI
Thesaurus [Internet]. ncithesaurus.nci.nih.gov. [cited 2023 Feb 23]. Available
from: https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&version=23.01e&ns=ncit&code=C149755&key=1151185279&b=1&n=null
3.
NCI
Thesaurus [Internet]. ncithesaurus.nci.nih.gov. [cited 2023 Feb 23]. Available
from: https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&version=23.01e&ns=ncit&code=C42993&key=501421394&b=1&n=null
4.
Kravetz RE. Suppository Mold. American Journal
of Gastroenterology. 2000 Jul;95(7):1817.
5.
Preparation of Suppositories | Pharmlabs [Internet]. pharmlabs.unc.edu.
Available from: https://pharmlabs.unc.edu/labexercises/compounding/suppositories/
6.
Annex 9
Guidelines on packaging for pharmaceutical products [Internet]. Available from:
https://cdn.who.int/media/docs/default-source/medicines/norms-and-standards/guidelines/regulatory-standards/trs902-annex9.pdf?sfvrsn=82b4c57d_2
7.
Packaging
Good Manufacturing Practices (GMPs) For Medicinal Products [Internet].
www.pharmaceuticalonline.com. Available from:
https://www.pharmaceuticalonline.com/doc/packaging-good-manufacturing-practices-gmps-for-medicinal-products-0001


Comments
Post a Comment